Who we are
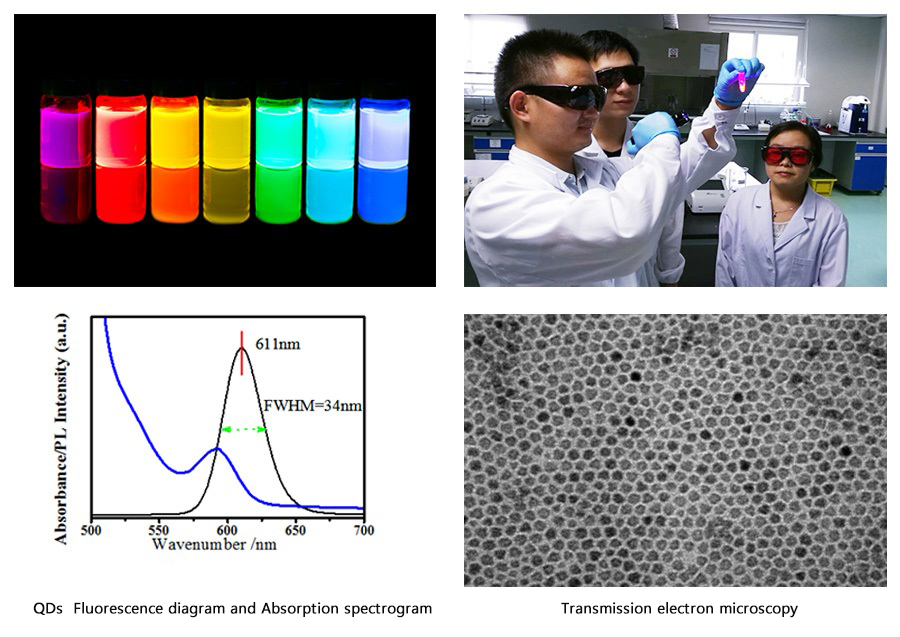
We are a vertically integrated company with R&D, manufacturing and commercial sales.We are a national hi-tech enterprise and CE certified. We have bulit ourself-owned core technologies with Ingenuity,and realized mass production of QDs diagnostic reagents with an annual output of over 2 million aliquots for the first time nationwide upgrading and promoting QDs tech.,we improved the precision of the chromatographic methodology to within 10% for the first time,making it the first IVD company to achieve the "pg" detection level using the chromatographic method in our country.
In the future, we will advance with time and continue to focus on the R&D of QDs technologies.
人才是企业的引擎
质量是企业立足的基石
客户是企业发展的伙伴
资本是企业腾飞的翅膀
开放合作,拥抱未来
争做最优秀的诊断试剂企业
打造最先进的体外检测平台
为社会提供精准检测
为客户提供优质服务
回报股东信任
助力个人发展
提供就业岗位
传播先进知识
传承中华美德
人才是企业的引擎
质量是企业立足的基石
客户是企业发展的伙伴
资本是企业腾飞的翅膀
开放合作,拥抱未来
争做最优秀的诊断试剂企业
打造最先进的体外检测平台
为社会提供精准检测
为客户提供优质服务
回报股东信任
助力个人发展
提供就业岗位
传播先进知识
传承中华美德
News
Development path
Professor Louis E. Brus first proposed the concept of colloidal quantum dots.
Professor Moungi G. Bawendi successfully synthesized quantum dots for the first time.
Jin Zhun quasi-quantum dots in Shenzhen love medical began to hatch.
Jin Zhun biological quantum point and students!
Quantum dot material Beidou system development platform, handheld instrument shock market.
R&D platform founded to make series of quantum dot material and put forward the new concept of whole-process quality control.
CE certified
Honor
CE-EN ISO13485
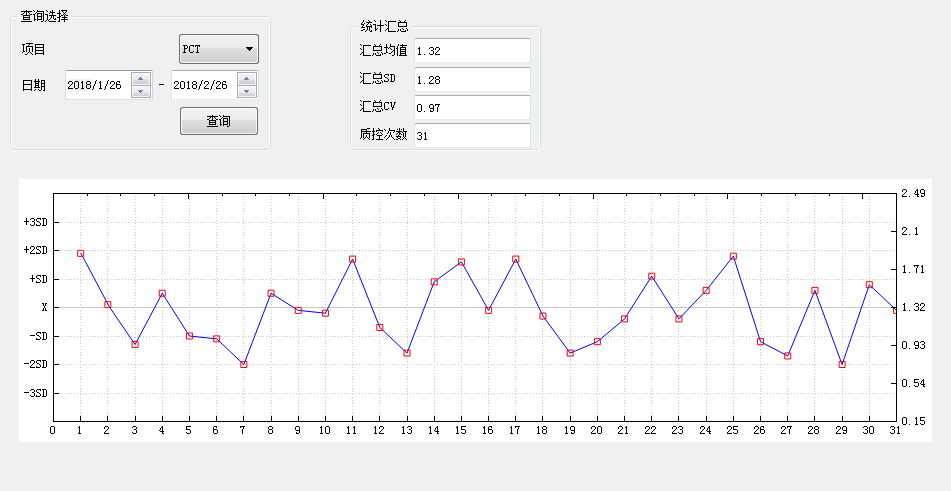
RIQAS 2018 PCT Certification
NCCL 2018 PCT Certification
Immunofluorescence quantitative analyzer
Procalcitonin quantitative assay kit (QDs immunofluorescence assay)
Member of Shenzhen Association of Medical Devices(2015)
Method for labeling immune globulin by quantum dots
Quantum dots labeled protein chip kit and preparation method
Immunitychromatography test card
Packing carton (Kingfocus quantum dots diagnostic reagent)
Immunofluorescence quantitative analyzer
Micro reaction chamber structure
Protein chip
CRP/PCT (C-reactive protein/procalcitonin) combined diagnosis test paper
Member of Shenzhen Medical Devices Quality Association
Member of Shenzhen Association of Medical Devices(2016)
Jointly diagnose test strip subassembly
Social responsibilities
Kingfocus Biomedical insists on developing rapid clinical diagnostic reagents based on QDs technologies.
Aiming to create the world's leading domestic brand,which features higher sensitivity, better accuracy, wider linear range, and more convenient operation,
To write a new chapter for human health!
hr@king-focus.cn